Goodbye Pelvic Exam? FDA Approves Self-Test for Cervical Cancer
From a very young age, we women have to submit to uncomfortable medical exams. One of our least favorite is the invasive pelvic exam. And although by now we’re used to it, there are still many women who skip follow-up exams to avoid the discomfort.
That’s why last week’s news from the U.S. Food and Drug Administration (FDA) has us celebrating. The FDA approved a new method of self-testing that we can do in the bathroom, USA Today reported.
What is cervical cancer self-testing?
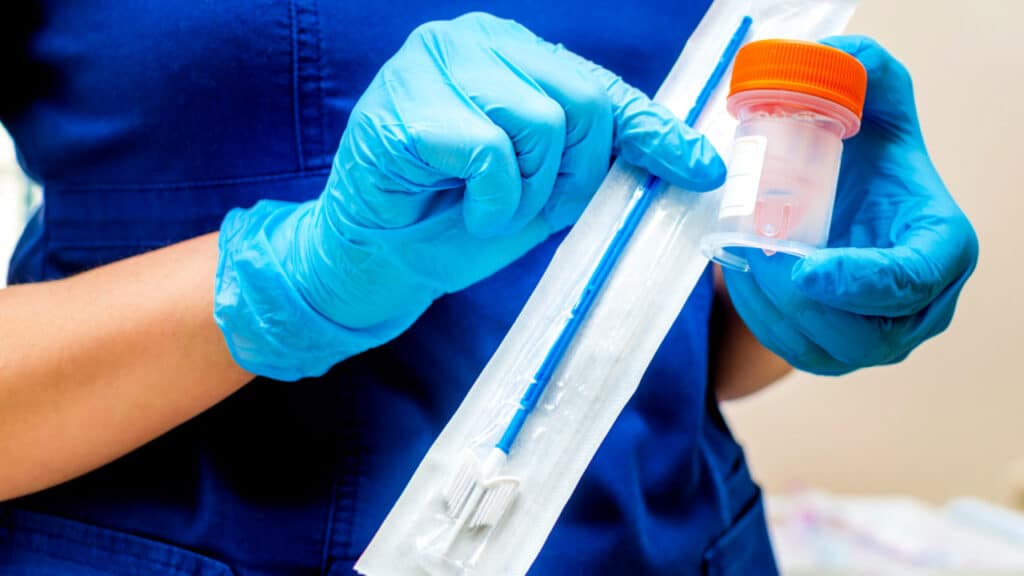
Gone are the bareback gowns, the doctor’s face between our legs and the speculum. Now, a doctor or pharmacist will hand us the self-obtaining swab that we simply take with us to the bathroom, similar to a routine urine test.
We simply insert the cotton swab into the vagina and swirl it three times. The doctor returns the completed test to the provider, who sends it to a laboratory for evaluation.
“It’s to reach women who traditionally haven’t been reached,” said Jeff Andrews, vice president of medical and scientific affairs at BD, one of the makers of the new method. Although the first trials are reserved for clinical use, BD plans to eventually offer an option for home use, Andrews said.
On the right track to eliminate cervical cancer
The FDA-approved tests report on 14 strains of HPV, the virus that causes 9 of 10 cases of cervical cancer. According to the CDC, nearly 200,000 women are diagnosed with a cervical precancer. Of those, 11,100 are diagnosed with cervical cancer caused by HPV. Annually, about 4,000 women die from cervical cancer.
Even more troubling, Latinas are 40% more likely to be diagnosed with cervical cancer and 30% more likely to die from cervical cancer.
However, this type of cancer can be prevented and cured if detected early. That’s why screening is critical. According to the CDC, most of the 13,000 new cases of cervical cancer diagnosed each year are in people who were not screened in the previous five years.
Caution: the test is not a substitute for a visit to the gynecologist
According to Andrews, the new FDA-approved test presents an opportunity for further advances in screening. Especially among those most at risk because they may not have regular access to gynecologic care.
“This cancer is unique,” Andrews said. “The goal with other cancers is early detection and treatment. The goal with cervical cancer is to find pre-cancerous cells, treat them and never have cancer.”
However, this does not replace the need to visit the OB/GYN annually. Although the exam reduces anxiety about a pelvic exam, “It doesn’t take the place of being able to talk about contraception, to talk about whether you’re in a safe relationship, to talk about your periods,” Dr. Anne-Marie Amies Oelschlager, an OB/GYN professor at the University of Washington and chair of the clinical consensus gynecology committee at the American College of Gynecologists and Obstetrics (ACOG), told USA Today. “It doesn’t take the place of breast cancer screening.”
Likewise, the test is not a substitute for the HPV vaccine. The preventive treatment initially targeted only girls and now women 11 and older can also get it. This vaccine prevents virus transmission but does not obviate the need for a screening test.